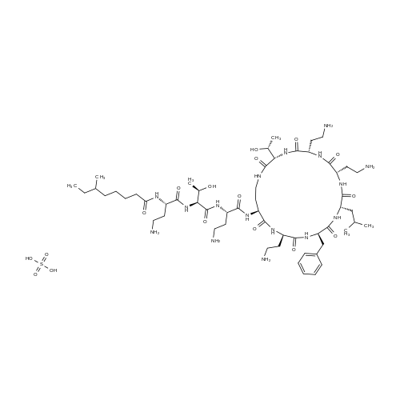
Description, clinical application and bioactivities of polymyxin B sulfate
General description
Polymyxin B sulfate and colistin, also known as colistimethate, have not been used for many years because less toxic antimicrobials are available. Gram-negative bacteria that are resistant to the aminoglycosides, β-lactams, and fluoroquinolones are becoming more common. These bacteria are often susceptible to the polymyxins [1]. Moreover, The polymyxins are amphipathic molecules that interact with lipopolysaccharide in the bacterial outer membrane. They have potent antiendotoxic properties and antibacterial activity against Pseudomonas aeruginosa and many of the Enterobacteriaceae [2]. Polymyxin B and colistin are usually given at a dose of 1.5–2.5 and 5 mg/kg/d, respectively, in two divided doses. Dosing must be altered in renal failure since the kidney is the primary route of elimination. Distribution into pleural fluid, joints, and cerebrospinal fluid is poor. Toxic effects involve the kidney and central nervous system. The polymyxins are recommended for serious systemic infections caused by gram-negative bacteria that are resistant to other agents [3].
Application
Common preparations of polymyxin B sulfate primarily include injections. It is used to resist the infection caused by gram-negative bacilli, mainly Pseudomonas aeruginosa, including urinary system infection, meningitis, lung infection, sepsis, skin, soft tissue, eye, ear, joint infection, etc. It also has a good therapeutic effect on the infection caused by other negative bacteria, such as aerogenes, Escherichia coli, pneumoniae and influenza bacilli [4].
Polymyxin B sulfate is an antibiotic. It has inhibitory effect on many negative bacteria such as pseudomonas aeruginosa, Escherichia coli, Klebsiella and Haemophilus, and is also sensitive to other antibiotic resistant strains. However, due to its serious renal toxicity and nerve blocking effect, it is only used for short-term rescue when burn complicated with sepsis, or for local spray flushing for external use [5]. This medicine should not be given intravenously in case of respiratory failure. The drug can be prepared with normal saline solution for external use.a
The antibacterial spectrum and clinical application of polymyxin sulfate B are similar to polymyxin e. it has inhibitory or bactericidal effects on gram-negative bacilli, such as Escherichia coli, Pseudomonas aeruginosa, paraescherichia coli, Klebsiella pneumoniae, acidophilus, pertussis bacilli and dysentery bacilli. Clinically, it is mainly used for infection caused by sensitive bacteria and Pseudomonas aeruginosa, as well as eye, trachea, meningitis, sepsis, burn infection and skin mucosal infection [6].
Pharmacological effects
Polymycin B sulfate has antibacterial action against pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, haemophilus, Enterobacter, Salmonella, Shigella, Bordetella pertussis, Pasteurella and vibrio gram-negative bacteria. Proteus, Neisseria, Serratia, Pruwitten, Gram-positive bacteria and obligate anaerobe were not sensitive to this class of drugs [8]. There was cross-resistance between polymyxin E and this drug, but there was no cross-resistance between this drug and other antibacterial drugs.
Polymyxin B sulfate is not absorbed orally. After injection, it is mainly excreted by urine, but only a small amount is excreted within 12 hours, and the concentration can reach 20-100 μg/ mL later. Drug excretion continues for 1 to 3 days after withdrawal. Polymyxin B sulfate is mainly used in wound, urinary tract, eye, ear, trachea and other infections caused by Pseudomonas aeruginosa and other pseudomonas, and can also be used for sepsis, peritonitis [9].
Polymyxin B sulfate is a cyclic cationic polypeptide antibiotic derived from Bacillus polymyxa that has the ability to bind and neutralize endotoxin. Studies have shown that systemic polymyxin B sulfate blunts the TNF-α response to endotoxin. Polymyxin B also blocks the formation of LPS-LPS binding protein complexes through its high binding affinity for the LPS molecule [10]. Unfortunately, infusion of polymyxin in humans results in nephrotoxicity and neurotoxicity, limiting its intravenous use to salvage therapy for Gram-negative enterobacteriaciae resistant to other antibiotics. Clinical trials of polymyxin B as an anti-sepsis/anti-endotoxin agent bound to dextran failed to advance through clinical trials in the 1990s, again related to systemic toxicity [11].
Indications
Polymyxin B sulfate is used to combat infections caused by Gram-negative bacilli, mainly Pseudomonas aeruginosa, including urinary tract infections, meningitis, lung infections, septicemia, and skin, soft tissue, eye, ear, and joint infections [12]. It also has a good therapeutic effect on the infection caused by other negative bacteria such as aerobacillus, Escherichia coli, pneumoniae and influenza bacilli, and the bacteria have complete cross-resistance to this product and polymectin E [13].
Usage and dosage in clinical practice
Polymyxins B sulfate has different dosage forms, different specifications of the usage and dosage may differ, please read the specific drug instructions, or follow the doctor's advice.
Polymyxins sulfate B for injection: for intramuscular or intravenous injection [14].
Polymyxins B sulfate for intramuscular injection: 10,000 to 20,000 units per kilogram of body weight per day. Inject in 3 times, dissolve with water for injection or sodium chloride injection.
Polymyxins B sulfate for intravenous infusion: 500,000-1,000,000 units per day, divided into 2 times, dissolved and diluted with appropriate sodium chloride injection or glucose injection.
Polymyxins B sulfate for intrathecal injection: 10,000-50,000 units per day for adults and 0.5-20,000 units per day for children, once every other day after 3-5 days, and the course of treatment is 2-3 weeks. Dissolve in proper amount of sodium chloride injection before use [15].
Side effects of polymyxin B sulfate
Nephrotoxicity: blood urea nitrogen and serum creatinine increased, occasionally renal failure and acute tubular necrosis. Neurotoxicity is also common. Temporary nervous system changes such as dizziness, dizziness, ataxia, slow speech, blurred vision, lethargy, insanity, limb numbness, abnormal taste, etc [16]. Large doses can cause neuromuscular blockade and respiratory arrest. Allergic reaction: allergic reaction can also occur, such as itching, rash, drug fever, and shock in severe cases. 5. Occasionally, it can cause leukopenia and hepatotoxicity. And intramuscular injection pain is obvious [17].
Matters needing attention
Polymyxin B sulfate damage to the kidney is more common, renal insufficiency should be reduced.
Polymyxin B sulfate intravenous injection may cause respiratory depression and is generally not used.
The intrathecal injection amount of polymyxin B sulfate should not exceed 5mg once to prevent the stimulation of meninges or nerve tissues.
Polymyxin B sulfate should not be combined with other drugs with renal toxicity or neuromuscular block to avoid accidents.
When polymyxin B sulfate injection is used to treat specific bacterial infections, patients should be informed that medication should be strictly followed, although they may feel better early on [18]. Incomplete dosing or incomplete treatment may reduce the efficacy of direct therapy and increase the development of resistance and render future polymyxin B and other antiblue drugs ineffective.
Diarrhea caused by antibiotics is a common problem, which is usually ended after drug withdrawal. Sometimes watery and bloody stools (with or without spasm and fever) occur after the antibiotic is initiated, and sometimes even two months or more after the last antibiotic is administered. If this occurs, the patient should contact a physician as soon as possible [19].
Patients with basic renal function should be tested before treatment. During treatment, renal function and blood drug level should be closely monitored to avoid the combination with curcumine muscle relaxant and other neurotoxic drugs (ether, curcumine, succinylcholine, galamine, trimethylammonium and sodium citrate), which may lead to respiratory arrest [20-22]. If respiratory paralysis symptoms occur, respiratory assistance should be given and drugs should be stopped. Like other antibiotics, the use of this drug may lead to the overgrowth of non sensitive bacteria, including fungi. If there is a double infection, an appropriate treatment plan should be formulated [23].
Drug interaction
Polymyxin B sulfate should not be used in combination with drugs that are harmful to kidney, skeletal muscle relaxants, aminoglycoside antibiotics, anesthesials with obvious muscle relaxation effects (such as enflurane), etc [24]. And quinine and magnesium should not be used intravenously at the same time. Polymyxin B sulfate combined with sulfonamides, rifampicin, semi-synthetic penicillin, etc. for the treatment of severe drug-resistant gram-negative bacteria infection, the effect is better than alone [25].
References
[1] A. Wang, W. Zhong, Y. Guo, L. Zhang, X. Zhang, J. Gao, Y. Shi, G. Mi, C. Wang, L. Chang, M. Li, L. An, L. Peng, X. Xie, Method for improving fermentation level of polymyxin B sulfate, North China Pharmaceutical New Drug R&D Co., Ltd., Peop. Rep. China . 2021, p. 9pp.
[2] T. Larosa, R. Davidson, D. Reid, Topical antibiotic, USA . 2021, p. 20pp.
[3] J.D. Jarrell, Metal oxide and polymer-controlled delivery systems, sunscreens, treatments, and topical coating applicators for antifungal and antimicrobial agents, Biointraface Inc., USA . 2021, p. 20 pp.
[4] S. Nakagawa, M. Shoji, Pharmaceutical composition and kit characterized by containing novel penam derivative or salt thereof and one or more compounds selected from β-lactamase inhibitor compound, antibacterial compound and salts of these, Fujifilm Corporation, Japan . 2021, p. 303pp.
[5] O.T. Skjerdal, T.M. Fagereng, A. Faegri, I.K.S. Cudjoe, Method for detecting and enumerating Listeria, Veterinaerinstituttet, Norway . 2021, p. 52pp.; Chemical Indexing Equivalent to 173:157130 (WO).
[6] E.L. Sastoque Cala, Compositions and methods for treating huanglongbing disease in citrus, New Life Crop Sciences LLC, USA . 2021, p. 93pp.
[7] M. Deng, J. Li, Y. Wang, H. Liu, L. Wu, M. Ye, X. Zhang, Z. Jiang, Q. Lin, H. Lin, Method for improving fermentation level of prodigiosin and application, Guangdong Industry Technical College, Peop. Rep. China . 2021, p. 11pp.
[8] J. Visvalingam, N. Yakandawala, S. Regmi, G. Guay, Coactive combinations of antimicrobials with dispersin B, Kane Biotech Inc., Can. . 2021, p. 155pp.
[9] J.T.G. Pena, J.M. Mitchell, H.L. Remmel, M.A. Morgan, Processes and compositions comprising cyclic peptide active agents for treating glaucoma, Aufbau Medical Innovations Limited, Ire. . 2021, p. 174pp.
[10] X. Zhao, K. Wang, J. Zhai, H. Du, Polyion complex nano material polypeptide carrier and its preparation method, Beijing Huaren Biotechnology Co., Ltd., Peop. Rep. China . 2021, p. 15pp.
[11] S. Ren, B. Niu, X. Hang, Eye ointment containing oxytetracycline hydrochloride for pets and preparation method thereof, Nanjing Lianzhi Medical Technology Co., Ltd., Peop. Rep. China . 2021, p. 9pp.
[12] R. Goodarzi, M. Taheri, F. Farahani, M. Roshani, B. Asghari, Comparative study of in vitro activities of polymyxin B commercial products on Pseudomonas aeruginosa isolated from hospitalized patients, Ars Pharm. 62(3) (2021) 270-279.
[13] K. Yang, Y. Peng, L. Wang, L. Ren, Polymyxin B engineered polystyrene-divinylbenzene microspheres for the adsorption of bilirubin and endotoxin, RSC Adv. 11(63) (2021) 39978-39984.
[14] D. Wei, Y. Liu, Z. Wang, R. Guo, Y. Jia, H. Yu, Optimization of quantitative determination of antimicrobial peptides by agar diffusion method, Shipin Yanjiu Yu Kaifa 39(17) (2018) 105-110.
[15] T.Z. Attia, M.A. Abdelmajed, M.A. Omar, K.M.B. El-Din, Selective Spectrofluorimetric Protocol for Determination of Commonly Used Gram-negative Bactericidal Drug in Combined Pharmaceutical Dosage Forms and Human Plasma, J. Fluoresc. 32(2) (2022) 603-612.
[16] L. Jin, H. Ding, V. Degirmenci, H. Xin, Q. Miao, Q. Wang, D. Zhang, Determination of the Peptide AWRK6 in Rat Plasma by Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) and Its Application to Pharmacokinetics, Molecules 27(1) (2022) 92.
[17] C.-H. Wang, J.H. Teoh, A.M. Thamizhchelvan, Y.H.J. Fuh, S.M. Tay, Hydrogels and methods of fabrication thereof, National University of Singapore, Singapore; Singapore Health Services Pte Ltd. . 2022, p. 124pp.
[18] B. Leng, W. Zhang, L. Zhang, G. Yan, X. Duan, Clinical Research Progress on Aerosolized Polymyxin in the Treatment of Pneumonia, Yiyao Daobao 40(8) (2021) 56-62.
[19] D.M.d.L. Oliveira, E.M.B. Souto, F.F. Padiha, L.N. Andrade, M.V. Chaud, P. Severino, P. Rezende, R.L. Cavalcante de Albuquerque Junior, R.d.S. Nunes, T.F.R. Alves, Method for obtaining polymeric membrane coated with nanoparticle for release of drugs for skin healing, Instituto de Tecnologia e Pesquisa, Brazil; Universidade de Sorocaba; Universidade Federal de Sergipe; Universidade Tiradentes . 2020, p. 30pp.
[20] E. Zhang, S. Gupta, E. Olson, P.R. Sinha, N.P. Hesemann, F.W. Fraunfelder, R.R. Mohan, Effects of Regular/Dilute Proparacaine Anesthetic Eye Drops in Combination with Ophthalmic Antibiotics on Corneal Wound Healing, J. Ocul. Pharmacol. Ther. 38(3) (2022) 232-239.
[21] R. Darlenski, J. Kazandjteva, N. Tsankov, Contact pemphigus: does it exist?, Eur. J. Dermatol. 31(6) (2021) 702-704.
[22] M. Meng, Y. Li, H. Yao, A method for screening and identifying antibiotic-resistant bacteria, Institute of Urban Environment, Chinese Academy of Sciences, Peop. Rep. China . 2022, p. 8pp.
[23] C.K. Burzell, Antimicrobial combination therapeutics including organophosphorous and organosulfurous compounds for treatment of microbial infections, Aequor, Inc., USA . 2022, p. 66pp.
[24] X. Zhang, Z. Huang, Y. Huang, C. Wu, Y. Zhou, G. Wang, Z. Zhai, Polymyxin B sulfate/raffinose dry powder inhalation and its preparation method and application as antibacterial drug, Jinan University, Peop. Rep. China . 2022, p. 15pp.
[25] S. Feng, P. Chen, Y. Zhang, F. Zhou, Y. Lu, Z. Deng, Y. Lu, Double layer and double releasing suppository for promoting wound healing after anorectal surgery and its preparation method, The First Affiliated Hospital of Xinxiang Medical University, Peop. Rep. China . 2022, p. 10pp.
You may like
Related articles And Qustion
Lastest Price from Polymyxin B sulfate manufacturers

US $5.00-0.50/KG2025-06-11
- CAS:
- 1405-20-5
- Min. Order:
- 0.10000000149011612KG
- Purity:
- 99% hplc
- Supply Ability:
- 5000kg

US $1.00/g2025-06-02
- CAS:
- 1405-20-5
- Min. Order:
- 1g
- Purity:
- 0.99
- Supply Ability:
- 20 tons